Description
STATLOCK Stabilization Devices are a more effective alternative to tape in helping improve clinical outcomes, quality of care and economic efficiencies.
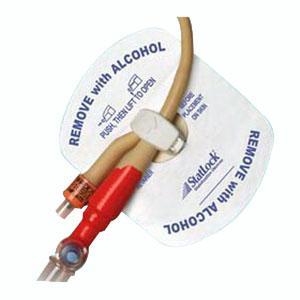
The STATLOCK Foley stabilization device accommodates latex 8-22 Fr. and silicone 8-26 Fr. catheters for the ultimate in versatility.
ADVANTAGES
- The STATLOCK Foley Stabilization Device sets new, worldwide standard for Foley catheter stabilization.
- Featuring a patented swivel retainer for enhanced patient comfort and convenience.
- Available in adult and pediatric sizes for most latex and silicone catheters.
- Releasable, lock-tight securement.
INDICATIONS FOR USE: The STATLOCK stabilization device is compatible for medical tubes and catheters.
CONTRAINDICATIONS: Known tape or adhesive allergies. Known sensitivity to benzoin. (Zinc Oxide PICC Neonatal Universal)
WARNINGS AND PRECAUTIONS
- Do not use the STATLOCK stabilization device where loss of adherence could occur, such as with a confused patient, unattended access device, diaphoretic or non-adherent skin, or when the access device is not monitored daily.
- Observe universal blood and body fluid precautions and infection control procedures, during the STATLOCK stabilization device application and removal.
- Suture the STATLOCK stabilization device pad to skin if necessary or deemed necessary.
- Avoid the STATLOCK stabilization device contact with alcohol or acetone, both can weaken bonding of components and the STATLOCK stabilization device pad adherence.
- Minimize catheter manipulation during STATLOCK stabilization device application and removal.
- Remove oil and moisturizer from targeted skin area.
- The STATLOCK stabilization device pad adherence and catheter/tube position should be routinely inspected.
- Orient the STATLOCK stabilization device so arrows point toward the catheter tip.
- The STATLOCK stabilization device should be replaced every 7 days.
- A STATLOCK stabilization device luer-lock connector must be used to secure venous and arterial catheters.
- Always apply the adhesive strip to central venous and arterial catheters at or near the insertion site when using a STATLOCK stabilization device
- This is a single use device. Reuse/and or repackaging may create a risk of patient or user infection, compromise the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure, and/or lead to injury, illness or death of the patent.
- Do not re-sterilize. The sterility of the single use device is not guaranteed following re-sterilization because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infection complications. Re-sterilization may compromise the structural integrity, essential material and/or design characteristics and may lead to an unpredictable loss of functionality and/or device failure.
- Special Patient Population
- Do not use alcohol or acetone containing products on pre-term infants
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
What is the delivery timeframe for in-stock items across Canada?
We offer 1-3 business day delivery for in-stock items across Canada.